Scientists from the PINK project partner Paris Lodron University of Salzburg (PLUS) have co-authored in a publication about the so-called NAVETTA system: “Application of the market-ready NAVETTA electrodeposition chamber for controlled in vitro exposure with nano-scaled aerosols”.
While the PINK project is primarily focused on in silico advancements, it still requires high-quality experimental data for validation, refinement, and regulatory acceptance. The NAVETTA system could act as a key experimental platform to generate reproducible inhalation toxicity data, bridging the gap between computational predictions and real-world biological responses.
This intersection highlights the complementarity of in silico and in vitro methods, reinforcing their combined potential to advance safe-by-design materials, alternative testing strategies, and regulatory science.
The scientific publication addresses the need for advanced in vitro inhalation exposure systems in the context of respiratory health, toxicology, and pharmacological research. Respiratory diseases such as COVID-19, chronic obstructive pulmonary disease (COPD), lung cancer, and tuberculosis remain significant global health threats. To assess chemical toxicity and air pollution risks, the scientific and regulatory communities are increasingly shifting from traditional in vivo models to in vitro approaches, supported by computational models (in silico). A key driver of this transition is the “3R” principle – Replacement, Reduction, and Refinement of animal testing. The European Union’s Directive 2010/63/EU highlights the necessity of replacing live animal testing whenever possible while maintaining safety and efficacy standards. Additionally, regulatory bodies such as the US Environmental Protection Agency (EPA) aim to phase out animal testing by 2035.
This study focuses on the NAVETTA exposure system, a novel electrodeposition-based in vitro exposure chamber designed to facilitate precise and reproducible aerosol deposition onto lung cell cultures. The system aims to bridge the gap between in vitro and in vivo data by improving the reliability and standardisation of in vitro inhalation toxicology studies. The study’s objectives include evaluating the NAVETTA system’s reproducibility and precision across multiple experimental conditions, demonstrating its ability to generate and deposit aerosols (both from liquid and dry states) under controlled conditions, and assessing its applicability for long-term in vitro inhalation studies, particularly in testing nanoparticle toxicity and pharmacological efficacy.
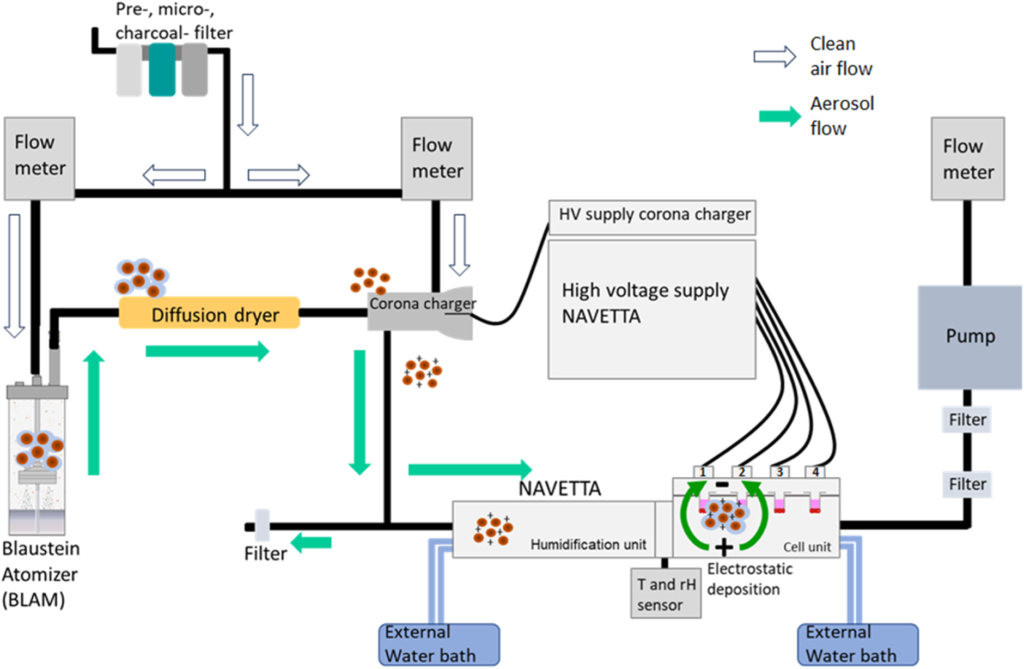
The NAVETTA system employs an air-liquid interface (ALI) exposure module that replicates real-life inhalation conditions more accurately than submerged cell culture models. It utilises computational fluid dynamics and physiologically based pharmacokinetic modelling to predict lung deposition patterns before exposure experiments. The system incorporates aerosolisation technologies, including collision-type atomisers for liquid aerosols and dry powder aerosolisers (PI and PX systems) for nanoparticle exposure. Furthermore, it employs electrodeposition technology to enhance uniform particle deposition onto lung epithelial cells. The study tested aerosol deposition of fluorescein aerosols (liquid-based) for assessing spatial deposition consistency and titanium dioxide (TiO₂) nanoparticles (dry powder) to investigate cytotoxicity and inflammatory response.
The findings demonstrate that the NAVETTA system exhibits high reproducibility, with less than 15% variability in deposition across experimental runs. The aerosols generated from both liquid and dry states fall within the respirable size range (PM2.5 and PM0.1), confirming their relevance for pulmonary exposure studies. Cell viability tests revealed that A549 lung epithelial cells maintained viability above 95% under typical exposure conditions for up to 24 hours. Exposure to TiO₂ nanoparticles resulted in a dose-dependent decrease in viability, confirming the toxic potential of these particles. Inflammatory response assessment indicated elevated IL-8 cytokine secretion following TiO₂ exposure, suggesting a pro-inflammatory effect, particularly at higher nanoparticle doses.
The study highlights several advantages of the NAVETTA system over existing models. The electrostatic deposition method enables more uniform and efficient aerosol deposition, reducing variability across test samples. Additionally, the system facilitates long-term exposure studies (up to 24 hours) while maintaining high cell viability. Unlike previous in vitro models, NAVETTA mimics realistic human lung exposure conditions, enhancing translatability to in vivo studies.
While the study successfully demonstrated the NAVETTA system’s reliability and reproducibility, several challenges remain. One major issue is regulatory acceptance, as no in vitro system, including NAVETTA, has been fully validated as a replacement for in vivo inhalation studies. Further collaboration with regulatory agencies is necessary to achieve widespread adoption. Future studies should also incorporate more advanced biological models, such as 3D lung models, co-cultures, and organ-on-a-chip technologies, to improve physiological relevance. Expanding research to evaluate a wider range of nanoparticles, pharmaceutical aerosols, and industrial pollutants would further extend NAVETTA’s applicability. Additionally, while the system currently supports up to 24-hour exposures, developing protocols for prolonged or repeated exposures could better replicate real-world inhalation risks.
In conclusion, this study provides a proof-of-concept for the NAVETTA exposure system, highlighting its potential to revolutionise in vitro inhalation research. By ensuring precise, reproducible, and realistic aerosol deposition, NAVETTA supports efforts to transition towards animal-free toxicity testing while maintaining regulatory and scientific integrity. Further validation and research will be crucial for achieving widespread acceptance in toxicology, pharmacology, and especially the SSbD approach and regulatory frameworks.
Parts of the research of this work (Magdalena Weiss, Benjamin Punz, Lisa Kleon, Vanessa Auer, Martin Himly) has been funded by the European Union`s R&I project PINK (grant agreement # 101137809).
Download the full paper here.