A scientist from the PINK project partner BASF was co-authoring in the following publication:
V. Malmborg, W. Wohlleben, D.A. Elam, V. Di Battista, J. Rissler, P.A. Clausen, U. Vogel, N.R. Jacobsen; Toxicity of carbon nanomaterials: A model to predict ROS production from easily measurable surface characteristics; Carbon, 234, 2025, 119997, https://doi.org/10.1016/j.carbon.2025.119997
The publication on carbon nanomaterial toxicity and the PINK project share a common focus on Safe-and-Sustainable-by-Design (SSbD) principles, particularly in assessing and mitigating risks associated with engineered nanomaterials. Both emphasize the role of physicochemical properties in determining material safety: the publication provides experimental insights into reactive oxygen species (ROS) production and toxicity mechanisms, while PINK develops computational models and decision-support systems for hazard prediction. Findings on surface chemistry-driven ROS generation could inform PINK’s in silico approaches, enhancing predictive toxicology frameworks for safer nanomaterial design.
The study investigates the oxidative stress potential of engineered carbon nanomaterials (CNMs) by assessing ROS production. Materials such as carbon black, graphene nanoplatelets (GNPs), and nanodiamonds are widely used in industrial applications due to their unique physicochemical properties. However, concerns regarding their toxicological impact, particularly oxidative stress and cytotoxicity, necessitate a deeper understanding of their interactions at the molecular and cellular levels.
The study examines 13 carbons (carbon black), 6 GNPs, and 3 nanodiamonds to identify physicochemical factors influencing ROS generation. ROS species, including superoxide anions (O₂•-), hydrogen peroxide (H₂O₂), and hydroxyl radicals (•OH), play a key role in oxidative stress-induced toxicity. The objective is to establish a predictive model linking ROS production to measurable properties such as specific surface area (SSA) and surface chemical composition, contributing to safer nanotechnology applications.
To assess ROS production, the study employs electron paramagnetic resonance (EPR) spectroscopy (bulk and spin-probe-assisted) and fluorescence-based assays (DCFH2-DA in acellular and cellular environments). These methods quantify both intrinsic and surface-related ROS generation, providing a comprehensive view of CNM oxidative potential.
Key factors influencing ROS surface production:
- SSA: Larger surface areas provide more reactive sites for oxidative interactions, though SSA alone does not fully explain ROS variability.
- Carbon sp²-hybridization: Higher sp² content, as in carbon black and GNPs, enhances ROS production by facilitating electron transfer reactions.
- Surface chemistry: Sulfur oxides (SOx) correlate with increased ROS production, while non-oxidized sulfur and oxygen content suppress it. SOx groups may enhance surface redox reactions.
- Surface functional groups: Oxygen-containing functional groups like quinones, carboxyls, and hydroxyls have been linked to ROS production. However, total oxygen content did not correlate positively with ROS activity, suggesting that specific oxygen groups or their abundance are more relevant.
- Material structure: Nanodiamonds, with predominantly sp³-hybridized carbon, exhibit significantly lower ROS production than graphitic materials, highlighting structural influence on oxidative reactivity.
- Surface contaminants/heteroatoms: Trace nitrogen and other elements detected via X-ray photoelectron spectroscopy (XPS) could alter ROS production by modifying electron transfer properties.
These findings suggest that ROS production is modulated by surface composition and chemical state rather than SSA alone, making targeted surface modifications a viable strategy for safer CNM design.
Key findings indicate that while ROS production correlates strongly with SSA, additional variability arises from surface chemical composition. Carbon blacks and GNPs exhibited a wide range of ROS activity, spanning nearly two orders of magnitude, whereas nanodiamonds showed significantly lower ROS production. XPS analysis revealed that carbon sp²-hybridization and SOx groups were major contributors to ROS production, while non-oxidized sulfur and total oxygen content had an inhibitory effect. A multiple linear regression model incorporating these variables achieved high predictive accuracy (R² = 0.99), enabling ROS estimation based on simple physicochemical properties.
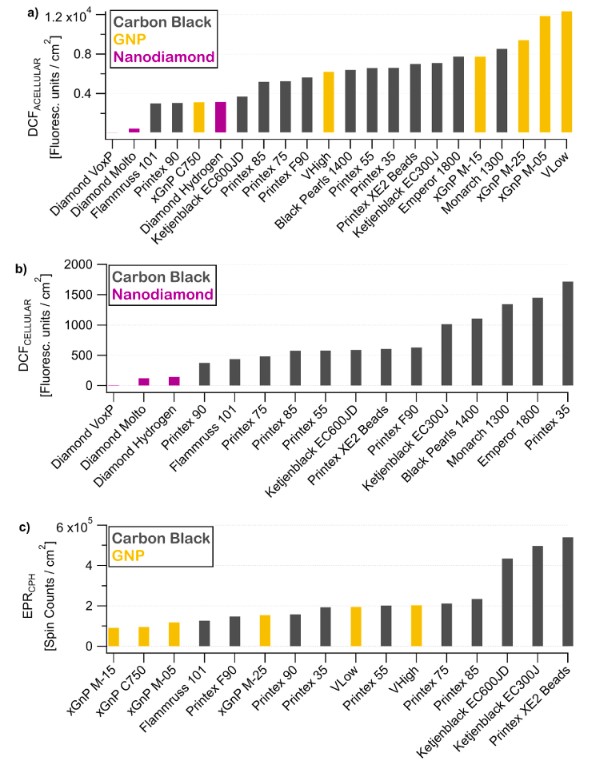
The study also examined the relationship between ROS production and cellular toxicity using hemolysis assays. Results showed a strong correlation (r = 0.96) between hemolytic potential and ROS activity, supporting the role of oxidative stress in CNM-induced toxicity. High-SSA materials with surface-bound SOx groups exhibited the most pronounced hemolytic effects, likely due to ROS-mediated lipid peroxidation.
Despite these advancements, several questions remain. The predictive model was developed for spheroidal and platelet-shaped CNMs, and its applicability to fiber-like nanomaterials such as carbon nanotubes is uncertain. Given the distinct toxicity mechanisms of high-aspect-ratio materials, further validation is required. Additionally, environmental transformations like oxidation, aggregation, and contamination could alter ROS production and toxicity, necessitating further investigation. The study focused on pristine materials, leaving the role of functionalization and surface coatings in modulating ROS activity an open question.
A critical next step is extending the study to in vivo models, as current research is limited to in vitro assays. While ROS generation and hemolytic potential provide valuable insights, biological responses in complex physiological environments may differ. Longitudinal studies on oxidative stress biomarkers, inflammatory responses, and genotoxicity in animal models are crucial for regulatory applications. As the nanotechnology industry advances, safer-by-design CNMs should be prioritized. Leveraging the predictive model developed in this study, researchers and manufacturers can optimize nanomaterial properties to minimize ROS production while maintaining desired functionalities. Future research should extend this model to emerging carbon nanostructures, such as graphene oxides and fullerene derivatives, refining risk assessment methodologies and enhancing nanosafety protocols
Follow this link to read the full publication.
Parts of the research of this work (Wendel Wohlleben) has been funded by the European Union`s R&I project PINK (grant agreement # 101137809).