Scientists (Giusy del Giudice, Laura Aliisa Saarimäki, Angela Serra, and Dario Greco) from the PINK project partner University of Helsinki (UH), were co-authoring in the following publication:
Maia MT, Fratello M, del Giudice G, Saarimäki LA, Möbus L, Serra A, et al. Nanomaterial grouping: Unraveling the relationship of induced mechanisms and potency at a temporal scale. Nano Today 2025; 61:102639. https://doi.org/10.1016/j.nantod.2025.102639
The publication proposes an advanced framework for grouping engineered nanomaterials (ENMs) based on mechanistic and temporal toxicogenomic evidence. The study integrates Adverse Outcome Pathway (AOP) theory, dose-dependent transcriptomics, and benchmark dose modeling to link molecular mechanisms of action (MOA) with ENM potency across diverse exposure settings. The authors develop a systematic approach to understand how ENM-induced biological responses evolve over time, with significant implications for hazard assessment, Safe and Sustainable-by-Design (SSbD), and regulatory decision-making.
The paper begins by noting that while ENM grouping is central to generalizing hazard across materials, “most strategies lack comprehensiveness in ENM and experimental settings.” Existing grouping approaches often rely solely on physicochemical similarities or single-endpoint toxicity assays. To overcome this, the authors leverage toxicogenomics, which “allows the characterization of the direct molecular mechanisms of action associated to ENM hazard.” Their central hypothesis is that AOP-directed MOA combined with ENM potency, i.e. “the dose required to activate a molecular response,” can enable a mechanistic, multiscale grouping of ENM. The study thus merges multi-dose transcriptomic data, dose-response modeling, and AOP enrichment analysis to identify shared biological responses and classify ENMs accordingly.
The analysis draws upon a curated database of 101 ENM exposure studies (Saarimäki et al., 2021), comprising 64 studies and 114 experimental conditions with multiple doses. These cover metal oxides (CuO, TiO₂), noble metals (Ag, Au), carbon nanotubes, silica, and polymeric ENM, tested in in vitro, ex vivo, and in vivo systems. Differentially expressed genes (DEGs) were filtered by fold-change (|log₂FC| > 0.58, FDR < 0.05) to identify significant transcriptional changes. Benchmark dose modeling (BMDx) was applied to derive transcriptional BMD (tBMD) and lower bound (BMDL) values, quantifying potency at which genes respond. “Genes whose BMDL values were outside the tested dose range or exhibited high uncertainty ratios were excluded” resulting in 10,842 dose-dependent DEGs (DD-DEGs) across 87 valid experiments. AOP-based enrichment was then performed using the AOP-Wiki ontology. Significant Key Events (KEs) and AOPs were identified via Fisher’s exact test (FDR < 0.05). The average BMDL of genes mapping to each KE served as a quantitative link between potency and biological scale (molecular → organismal). Consensus hierarchical clustering and machine learning (XGBoost with SHAP feature importance) were used to identify key drivers of grouping.
Exposure duration strongly influenced the type of molecular response. “Genotoxicity-related AOPs were found triggered at longer exposures,” whereas higher ENM potency correlated with shorter exposures and early molecular events. Conversely, “lower potency was linked to prolonged exposures and advanced stages of biological processes.” Short-term (29 studies) and intermediate-term (18 studies) exposures displayed distinct mechanistic profiles: acute exposures emphasized oxidative stress and inflammation, while intermediate-term exposures showed DNA damage and repair-related KEs. The authors note that “time does not affect the progression of events in the AOP framework, but potency does”, suggesting that dose sensitivity, rather than time per se, dictates how biological events unfold across scales.
By stratifying KEs into potency categories, the authors observed that “KEs with lower triggering doses were associated with basic molecular and cellular initiating events, while higher doses corresponded to advanced tissue and organ-level outcomes.” This implies that potent ENMs induce early molecular disruptions rapidly, whereas less potent ones require prolonged or higher exposures to reach adverse outcomes.
Consensus clustering identified five mechanistic clusters at short-term and three at intermediate-term exposures. For example:
- Clusters 1–2 (short-term): TiO₂ and carbon ENMs elicited inflammatory KEs, consistent with “recruitment of inflammatory cells and secretion of pro-inflammatory mediators”
- Cluster 3: Enrichment of glucocorticoid receptor-related KEs, indicating potential endocrine disruption
- Cluster 4: Fibrotic responses driven by “collagen accumulation and angiogenesis,” matching known MWCNT-induced lung fibrosis
- Cluster 5: Predominant apoptosis-related pathways in copper and silica ENMs
At intermediate durations, clusters reflected systemic metabolic and immune alterations – iron-silica ENMs perturbed “lipid metabolism, xenobiotic response, and oxidative stress,” while copper-based ENMs were linked to fibrosis and pulmonary remodeling.
Feature attribution via SHAP analysis demonstrated that grouping is primarily influenced by chemical composition, size-dependent parameters (diameter, surface area, charge), and biological system. “Both intrinsic and extrinsic nanodescriptors, representing size and colloidal stability, were predictive of grouping,” along with descriptors of bio–nano interface interactions (e.g., glycine or ester adsorption energies).
Importantly, “features were interdependent and differed in quantity and connectivity, indicating differences in the response dynamics.” Network analyses revealed stronger feature interconnections at short-term exposures, reflecting rapid, transient molecular changes, while longer exposures showed fewer but more stable interactions – consistent with adaptive or systemic processes.
The study demonstrates that integrating dose–response potency, AOP linkage, and transcriptomics yields a quantitative, mechanistic classification of ENM hazards. The authors emphasize that their approach “paves the way for the construction of quantitative AOPs and holds significant implications for ENM hazard assessment and regulatory decision-making.”
They argue that this framework complements New Approach Methodologies (NAMs) and SSbD paradigms, enabling data-driven hazard prediction without extensive animal testing. By “accounting for multifactorial exposure characteristics beyond chemically centered factors” it offers a holistic view of nanomaterial–biological interactions across temporal and organizational scales.
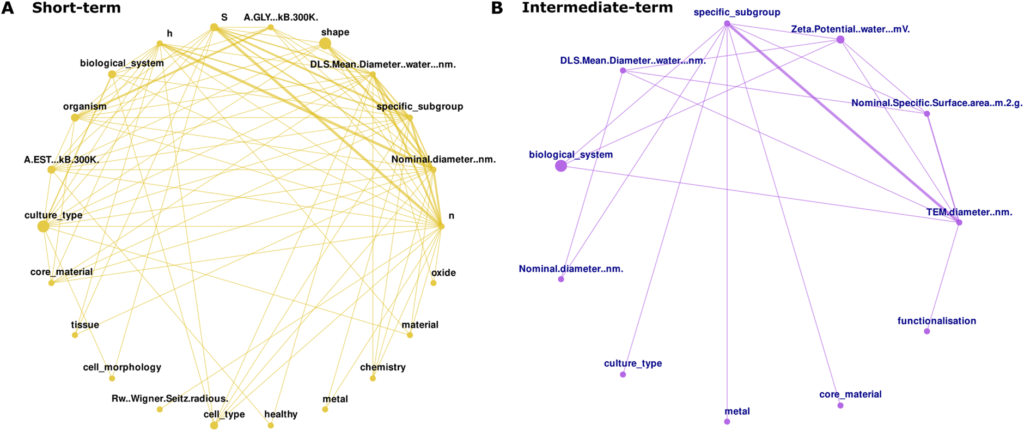
Features relevant for the grouping exhibit intricate relationships and reflect distinct response dynamics. Feature interactions were extracted and averaged based on 50 model’s prediction ensuring a minimum of 60 % of data completeness. A) at short-term and B) intermediate-term. ENM features are represented by nodes and relationships between features are represented by undirected edges. Node size refers to the average feature contribution of the ENM feature and edge thickness, the strength of the interaction between ENM features (Figure taken from publication)
Maia et al. provide a robust, systems-level methodology for grouping nanomaterials based on mechanistic toxicogenomics. Their findings establish that: a) Exposure duration and potency jointly shape ENM-induced biological mechanisms; b) Genotoxic and inflammatory pathways are temporally distinct; and c) Grouping can be predicted by integrating ENM physicochemical features, potency, and biological system descriptors. Ultimately, this study advances the scientific and regulatory capacity to generalize ENM hazard through mechanistic evidence, aligning toxicogenomics with quantitative AOP modeling, NAM-based risk assessment, and EU SSbD frameworks.
This work introduces a novel mechanistic and potency-based strategy for grouping nanomaterials, combining dose-dependent transcriptomics with the AOP framework to capture temporal and multiscale biological responses. Its key achievement is the first evidence-based classification of ENMs by mechanistic similarity, revealing how exposure duration and potency jointly determine hazard progression, thereby advancing regulatory grouping and SSbD approaches.
Follow this link to read the full publication.
Parts of the research of this work (University of Helsinki) has been funded by the European Union`s R&I project PINK (grant agreement # 101137809).